

The Greek philosophers of V century b.C. gave the most disparate and fanciful answers to
the fundamental questions about the principle of the physical world:
According toTalete the universal principle is water, whereas according to Anassimene is
air.
According to Empedocle of Agrigento the fundamental principles are four: water, air, earth
and fire.
According to Anassagora of Clazomene, who was the founder of the first Athenian
philosophical school, all the bodies are made of seeds (parts of the same kind, according to Aristotle
), that have a different nature according to the substances ( metals, stones, living
beings ) and are divisible in more and more smaller (infinitesimal) parts.
The first phylosophers speaking about atoms ( from the Greek word atomos = indivisible )
were Leucippo of Mileto and Democritus of Abdera, who affirmed the discontinuity of
matter.
Within the atomic theory of Leucippo and Democritus, which conflicts with the continuous
divisibility of Anassagora's seeds, atoms are considered to be falling in vacuum subjected
to their weight, and to be joining among each other to form bodies.
After over 2200 years, at the beginning of the XIX century, the English chemist and
physicist John Dalton, by studying the laws of chemical combinations, showed the necessity
to take back the atomic theory to explain the constant ponderal relationships among the
amounts of elements forming chemical compounds.
Dalton's studies were subsequently deepened at by the chemist and physicist Amedeo
Avogadro (Turin, 1776-1856 ) and by the chemist Stanislao Cannizzaro (Palermo, 1826-1910
), that founded the modern theoretical chemistry, furnishing solid bases to the
development of the physical-chemical knowledges during XX century.
So began the development of atomic physics, that in last years of XIX century received a
decisive impulse by the researches on cathode rays and X-rays.
The first, clear and decisive test of the corpuscular nature of matter was achieved in
1897 by J.J. Thomson, when he discovered electron.
The exploration of microcosm began in the second half of the nineteenth century with
the study of the electric discharge in rarefied gases.
Some laboratory devices were already available,the so-called Ruhmkorff coils,able to
generate high voltages,about a few tens of KV,and based on the electromagnetic induction
phenomenon,that was discovered by Faraday.
Therefore a lot of experiments was made on the characteristics of the electric discharge
in pipes (the Crookes pipes) containing a rarefied gas, kept at a pressure of a few ten
thousandths of one mercury millimeter, and several physicists studied the characteristics
of the so-called "cathode rays ",that were emitted by the cathode of the
discharge pipes.
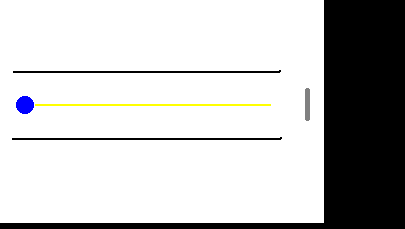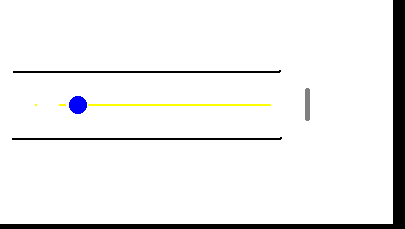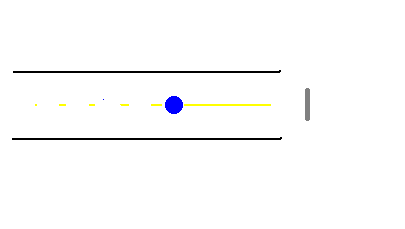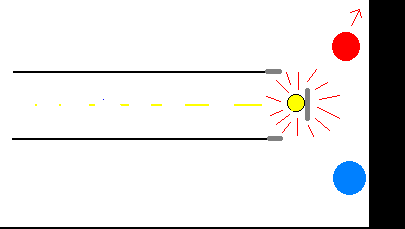
Cathode rays were observed to be able to generate some heat in collisions against a
metallic plate disposed on their trajectory in a discharge pipe,and to be deflected either
by an electric field applied to them by using a suitable discharge pipe,the so-called
Braun's pipe,across a pair of metallic plates,or by a magnetic field produced by magnets
or electromagnets disposed near the discharge pipe.
Cathode rays were also observed to propagate on a straight line from cathode to anode, in
absence of both electric or magnetic fields,and to produce a greenish fluorescence by
impact against the glass wall in front of cathode.
While studying experimentally cathode rays,Roentgen, a German physicist working at
Karlsruhe University (Nobel prize winner in 1901), discovered a new type of rays,he
denominated X-rays for their mysterious nature, that are produced by the strong
deceleration of cathode particles in collisions against the glass of the pipe.
It is known in fact from electromagnetic theory that an electric charge which is subjected
to an acceleration or a deceleration, radiates electromagnetic waves.
X-rays, differently from cathode-ray particles,are deflected neither by electric fields
nor magnetic fields.
Therefore, as it was shown by several experiments, X-rays are electromagnetic radiations
with a wavelenght among some billionths of centimeter and some hundred millionths of
centimeter.
Roentgen discovered X-rays by chance,while observing the fluorescence induced by them in
barium salts, and observed besides their main property of impressing photographic plates.
Becquerel, a French physicist, Nobel prize winner in 1903, discovered the natural
radioactivity, by observing uranium salts emit a radiation which is able to impress
photographic plate.
He discovered, in particular, by making experiments with a magnetic field,that uranium
salts emit three kinds of radiation , that were called a,
b and g-rays.
The first two exhibit a corpuscular nature,whereas the latest one exhibits a wave-like
nature.
In the immediately following years other physicists discovered that a particles have a positive electric charge , while the b ones have a negative electric charge.
It was shown besides that g-rays , which are able to go beyond a very thick layer of lead,
are electromagnetic waves with a wavelength much smaller than the one of X-rays.
Mr. Curie and Mrs. Sklodowska-Curie, Nobel prize winners together with Becquerel,
discovered radium, which is a new chemical element that emits a radioactivity much more
strong than the one of uranium, from which it derives by radioactive decay across a family
of radioelements, whose founder is uranium.
It was discovered besides that the radioelements that are extracted from the rocks of the
earth's crust, belong to three radioactive-elements families, whose founders are uranium,
thorium and actinium and which end with non decaying elements, that are isotopes of lead.
In other words, on the Earth, in a time of about five billions of years , from the founder
elements of each family, by emission of a and b particles and of g
-rays, have been produced, in equilibrium conditions, all the following radioactive elements, till the
latest one, thate is lead.
In fact, by determining the today concentrations of some elements in earth rocks and by
measuring the decay speed of each element, it is possible to calculate the age of Earth.
Among the fundamental physics discoveries that characterize the nineteenth century, we
remember the measure of the ratio between the electric charge and the mass of the
cathode-ray particles, that was made in 1897 by the English physicist Joseph John Thomson
( Nobel prize winner 1906 ) ,who showed definitely their particle-like nature, coining the
term "electron" for the first particle identified as a fundamental component of
atoms which form matter.
We can imagine atoms are like microscopic planetary systems.
That is a sentence frequently used to explain by an analogy the structure of the atom, if
we compare the sun to atomic nucleus, whose mass is nearly equal to the one of the whole
atom, and the planets orbiting about the sun to the electrons orbiting about nucleus.
We know that nuclei contain some fundamental particles, which collectively are named
nucleons, that is protons and neutrons: the first ones are about 1840 times more heavy
than electrons and have a positive electric charge, the second ones have a mass nearly
equal to the one of protons and are electrically neutral .
Electrons orbiting about nucleus have a negative electric charge equal to the one of
proton , form the "shell" of atoms and determine, by electromagnetic forces, the
mechanical, thermal, chemical and electromagnetic properties of chemical
elements,(metals,semiconductors and metalloids ) in the three aggregation states (solid,
liquid, and gaseous ).
In thermodynamics we define as a black body an ideal body which is able to absorb all
the thermal radiation incident on it and, vice-versa, is able to emit , with the maximal efficiency, the thermal
radiation characteristic of its absolute temperature (in Kelvin degrees ).
A black body consists practically of a container with a reflecting intern surface, which
is maintained at a constant temperature and has a small hole across which it emits or
absorbs thermal radiation (infrared radiation),whose continuous spectrum can be examined
with suitable spectroscopes equipped with special lenses and prisms, able to transmit
infrared radiation, and with infrared-sensors generating an electric signal whose
amplitude is proportional to the intensity of the infrared radiation.
A theoretical problem of great importance concerned, in the latest years of the nineteenth
century, the research of a law that were being successful in deriving the experimental
data on the optical and X-rays spectra.
Several physicists, as, for example, Rayleigh, Jeans and Wien, developed some incomplete
theories, based on the classical electromagnetism and on thermodynamics, but nobody of
them succeeded in finding a theoretical solution which might be complete and definitive.
In 1900 an innovative solution was furnished by the German physicist Max Planck (Nobel
prize winner 1918 ), who for that is considered the father of quantum theory.
Planck hypothesized for the first time the quantization of the electromagnetic energy of
the harmonic oscillators representing the electric charges moving as harmonic oscillators.
He calculated the emission power of a black body mantained at a constant temperature, by
considering the absorbed or emitted energy from each harmonic oscillator,as a multiple of
an elementary quantity and E = hf, where f is the frequency of the emitted or absorbed
electromagnetic waves and h is an universal constant, the so-called Planck's constant.
Only by introduction of this hypothesis Planck was able to resolve definitively the
problem of the black body, by giving the formulae in accordance with the experimental
data.
His work was the beginning for the development of a new physics, the quantum physics.